Although wet chemistry looks outdated, it can be useful in an initial assessment of ore, especially under field conditions. And yes, a handheld XRF still costs 35k. So why bother to prepare samples for the microscope when a simple test shows that there is no nickel anyway? Or determining zinc within seconds even underground in a mine? I have compiled a few tests here which I use regularly for zinc, copper, nickel and cobalt.
Disclaimer: I hereby disclaim any and all responsibility and liability for any damages that have been caused by or in connection with the use of this site. Safety for your health and life is your responsibility alone.
a) Test for Secondary Zinc Minerals (“Zinc Zap”)
This test is a famous one and was used as a field test with large quantities of chemicals sprayed into the landscape. Today safety awareness rises and many companies banned the use of Zinc Zap due to the toxic nature of N,N-diethylaniline. Nevertheless, the test is extremely useful as a spot test with very low amounts of testing solutions.
The test is good for secondary zinc minerals (such as smithsonite, hydrozincite, hemimorphite, saucontite) and willemite; less staining is apparent on unweathered sphalerite, zincite, or franklinite; it works best in the absence of iron sulfides as iron sulfate will also react to produce a deep blue color that will mask any red from zinc; best used in carbonate terranes. Once mixed, solution deteriorates within several hours, depending on temperature; chemicals are light sensitive—store individual solutions separately in light-proof bottles
Solution A: 3% potassium ferricyanide in water
Solution B: 3% oxalic acid, 0.5% N,N-diethylaniline in 1% HCl (i.e., 9 ml concentrated HCl, 30 g oxalic acid, 5 mL diethylaniline in 1 L water)
Mix equal portions of A and B into a plastic spray bottle and spray onto a rock or mineral; alternately, mix the two solutions on the sample to be tested; positive test is a bright red stain (test on galvanized pail)
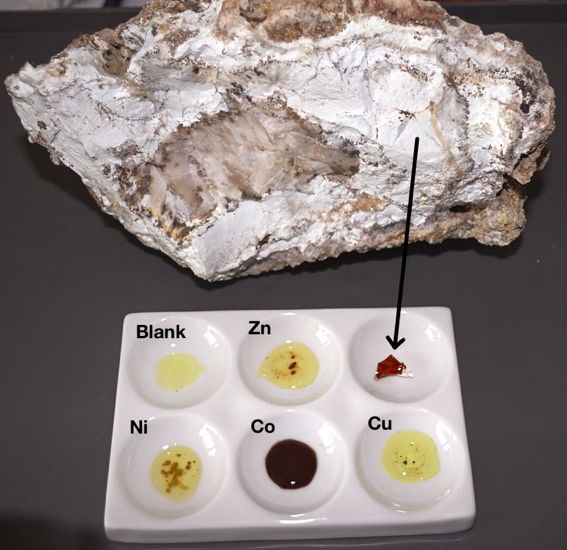
Here is an example how it looks with non-sulfide zinc ore from Italy (Zorzone), and some salts of nickel, cobalt and copper for comparison. The potassium ferricyanide alone reacts with iron(II) and the cobalt salt, but the color is different from the Zn one. "Blank" are just the two solutions without sample. The red of the zinc is more colorful than captured on the fotos.

For a nice example see also the Zorzone section: Zorzone banded ore
b) Test for copper, nickel, cobalt
This test procedure gives a qualitative determination of copper, nickel and cobalt in one run. The ore is grinded to powder, then mixed with sodium hypochlorite solution and a few drops of hydrochloric acid in a thick-walled gas-tight container. Close the lid immediately after addition of the acid. The idea is to produce nascent chlorine which is extremely aggressive and dissolves even the more difficult ores. Be sure you open the container afterwards in a well ventilated area.
Divide the supernatant into two aliquots and add some solid sodium fluoride, potassium thiocyanate (KSCN) solution and then some acetone to the first one. If a blue color appears, the ore contains cobalt. The sodium fluoride masks iron, while thiocycanate forms a complex with the Co-ions. Acetone is used here to lower the concentration of water while still providing a polar enough solvent for the ionic solutes. The competition between water and thiocycanate for coordination of the cobalt(II) favors thiocyanate more strongly in aqueous acetone than in pure water because of the lower water concentration.
Take the second aliquot and add enough ammonium hydroxide solution (25%) to make it alkalic. If the sample contains iron, a brown precipitate will form. Let it settle. If the supernatant is blue, the ore contains copper which forms a blue complex with ammonia. Take the supernatant off with a pipette or decant it, make sure the pH is well over 8 and add a few drops of a saturated solution of diacetyldioxime in ethanol to it. If a red precipitate forms, the ore contains nickel. If it just gets a little red, than it does not contain nickel. If the ore contains a lot of copper, then use sodium hydroxide instead of ammonium hydroxide solution to avoid formation of the blue complex.
Summary
Ore powder + sodium hypochlorite solution + hydrochloric acid
=> let settle for some hrs, take supernatant, divide in two aliquots
1) add to the first aliquot: solid sodium fluoride, KSCN solution, acetone -> blue color = cobalt
2) add to the second aliquot: ammonium hydroxide solution until alkalic
=> let the iron precipitate settle, take supernatant
a) supernatant is blue -> ore contains copper
b) add diacetyldioxime solution -> red precipitate = nickel
Fe-Cu ore from Miggiandone, Italy. The copper forms the characteristic blue complex while the iron precipitated.

Ni-Co-As ore from the Val d'Anniviers in Switzerland. Left side the test result for cobalt, in the middle the test for nickel and on the right a piece of the ore.

References
Bloom, H.: Field methods for the determination of nickel using dimethylglyoxime. Economic Geology 57 (1962) 595-604
Hitzmann et al: Classification, Genesis, and Exploration Guides for Nonsulfide Zinc Deposits. Economic Geology 98 (2003) 685–714
Kärner, K.: The metallogenesis of the Skorpion non-sulphide zinc deposit. PhD thesis, 2006.
Feigl, F.: Spot tests in inorganic analysis. Elsevier, 1958.
Schuster, N.: Mineralbestimmung durch einfache chemische und physikalische Methoden. Chr. Weise Verlag, 2007


